Dye-based qPCR & RT-qPCR
One disadvantage of intercalating dye-based methods is that they detect any dsDNA produced in the reaction. This includes off target amplification products and primer-dimers, which results in inaccurate quantification. Therefore, it is imperative to perform a denaturation (melt) curve after each qPCR experiment to verify reaction specificity and ensure that only the desired target was amplified. Additionally, unlike with probe-based qPCR that permits multiplexing, only one target can be interrogated at a time in a dye-based qPCR experiment.
TaqMan® is a registered trademark of Roche Molecular Systems, Inc.
SYBR® is a registered trademark of Molecular Probes, Inc.
Videos
-
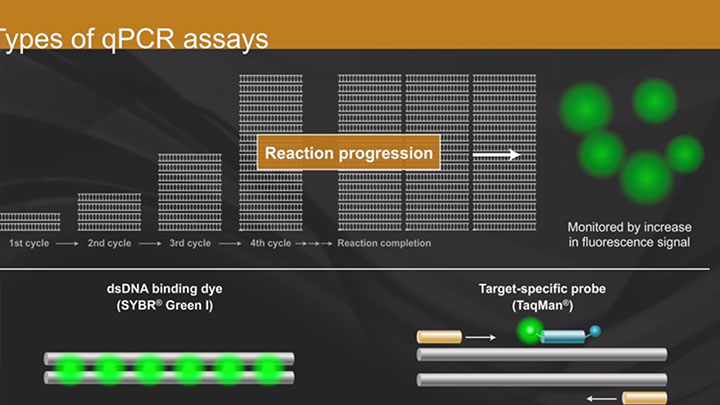
Overview of qPCR
Learn the basics of qPCR in this short animation.
-
Learn how we analyzed plates and plates of qPCR data, and how Luna stacks up to the competition.

