DNA Preparation
Choose Type:
- Affinity Purification and On-column Cleavage (E6901)
- Comet Assay - Modified for Detection of Oxidized Bases Using the Repair Endonucleases Fpg, hOGG1 and Endonuclease III (Nth)
- Construction of the Fusion Plasmid (E6901)
- Fusion Protein Expression (E6901)
- Preparation of Media and Solutions (E6901)
- Primer Design for Restriction Enzyme Cloning (E6901)
- Simplified Expression and Purification Protocol (E6901)
- Optimizing Restriction Endonuclease Reactions
- Fusion Constructs (E6901)
- Protocol for Cre Recombinase (M0298)
- Protocol for Direct Digestion of gDNA during droplet digital PCR (ddPCR)
- Protocol for generating 32 bp fragments from modified CpG sites in genomic DNA using MspJI, FspEI or LpnPI
-
Why Choose the K. lactis Protein Expression Kit?
Review the advantages of the K. lactis Protein Expression Kit for rapid, high yield protein expression in yeast.
-
A Modern Day Gene Genie Sir Richard Roberts on Rebase
- Molecular Cloning Technical Guide
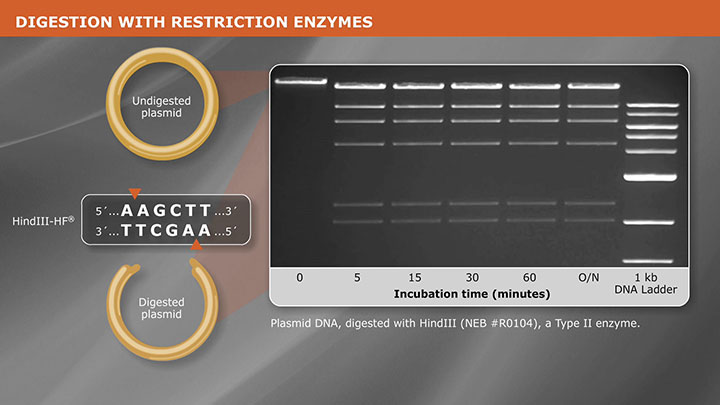
- Cleavage Of Supercoiled DNA
- Compatible Cohesive Ends and Generation of New Restriction Sites
- Dam-Dcm and CpG Methylation
- Frequencies of Restriction Sites
- Recleavable Blunt Ends
- Recleavable Filled-in 5' Overhangs
- Why Choose Recombinant Enzymes?
- Troubleshooting Guide for Cloning
- Activity at 37°C for Restriction Enzymes with Alternate Incubation Temperatures
- Alteration of Apparent Recognition Specificities Using Methylases
- Cleavage Close to the End of DNA Fragments
- Digestion of Agarose-Embedded DNA: Info for Specific Enzymes
- Double Digests
- Heat Inactivation
- NEBuffer Activity/Performance Chart with Restriction Enzymes
- Optimizing Restriction Endonuclease Reactions
- Restriction Endonucleases - Survival in a Reaction
- Restriction Enzyme Diluent Buffer Compatibility
- Site Preferences
- Star Activity
- Traditional Cloning Quick Guide
- cDNA/Reverse Transcriptase Tips
Feature Articles
Brochures
Selection Tools
Troubleshooting Guides
Usage Guidelines
- Shah, S., Sanchez, J., Stewart, A., et al. (2015) Probing the Run-On Oligomer of Activated SgrAI Bound to DNA PLoS One; 10(4), PubMedID: 25880668, DOI: 10.1371/journal.pone.0124783.
Products and content are covered by one or more patents, trademarks and/or copyrights owned or controlled by New England Biolabs, Inc (NEB). The use of trademark symbols does not necessarily indicate that the name is trademarked in the country where it is being read; it indicates where the content was originally developed. The use of this product may require the buyer to obtain additional third-party intellectual property rights for certain applications. For more information, please email busdev@neb.com.
This product is intended for research purposes only. This product is not intended to be used for therapeutic or diagnostic purposes in humans or animals.