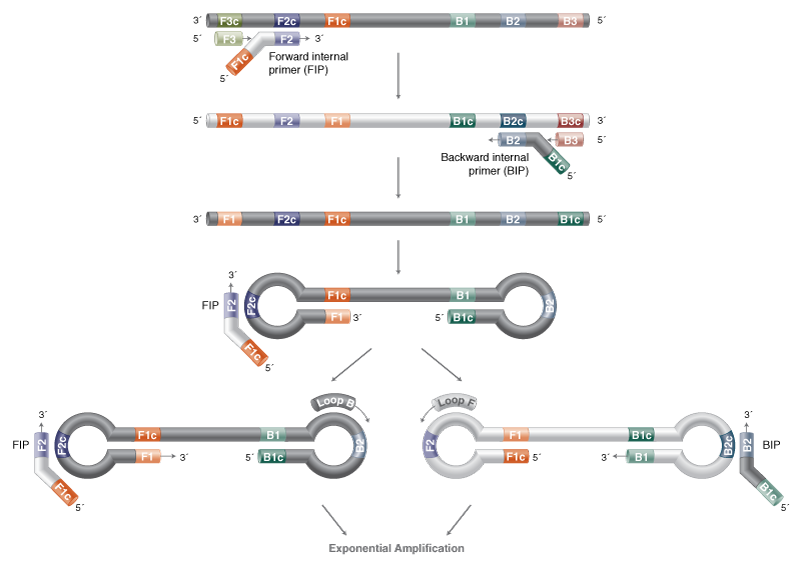
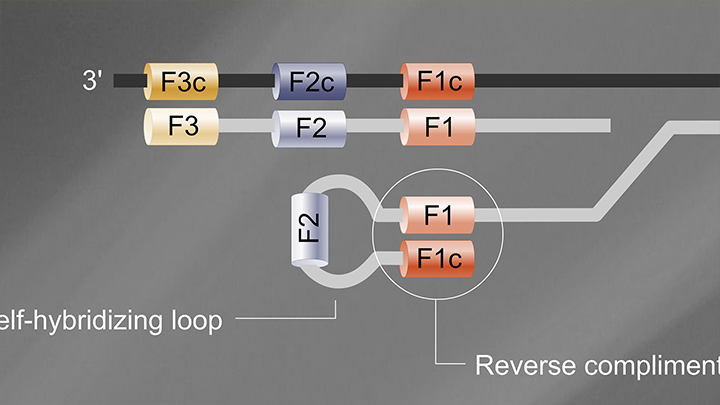
Loop-Mediated Isothermal Amplification
Choose Type:
- How can I see the products of a LAMP reaction?
- How do I use Tte UvrD Helicase for reducing non-template amplification in LAMP reactions?
- Does NEB have a master mix for LAMP or RT-LAMP reactions?
- What is the difference between Bst DNA Polymerase, Large Fragment, Bst 2.0, and Bst 3.0 DNA Polymerase?
- How do I use WarmStart® RTx in RT-LAMP?
- How do I use Antarctic Thermolabile UDG for carryover prevention in LAMP reactions?
- Loop-mediated Isothermal Amplification (LAMP)
- WarmStart LAMP Kit (DNA & RNA) Protocol (E1700)
- WarmStart Colorimetric LAMP 2X Master Mix Typical LAMP Protocol (M1800)
- Protocol for LAMP Reactions with Tte UvrD Helicase (#M1202)
- Protocol for unwinding double stranded DNA with Tte UvrD Helicase (#M1202)
- Typical LAMP Protocol (M0275)
- Typical LAMP Protocol (M0538)
- Typical LAMP Protocol (M0374)
- Typical RT-LAMP Protocol
- WarmStart® Multi-Purpose LAMP/RT-LAMP 2X Master Mix (with UDG) Protocol (NEB #M1708)
- WarmStart® Fluorescent LAMP/RT-LAMP Kit (with UDG) Protocols (NEB #E1708)
- WarmStart Colorimetric LAMP 2X Master Mix with UDG Typical LAMP Protocol (NEB #M1804)
- SARS-CoV-2 Rapid Colorimetric LAMP Detection Assay Protocol (NEB #E2019)
-
Mitigating Risk and Ensuring Consistent Supply Chain Through Internal SARS-CoV-2 Testing with RT-LAMP
This feature article describes how NEB scientists established a CLIA-certified SARS-CoV-2 onsite testing program utilizing saliva and colorimetric loop-mediated isothermal amplification (LAMP)
- Isothermal Amplification Brochure
- NEB LAMP Primer Design Tool
- Amplification Reagents for Molecular Diagnostics Applications (2017)
- Colorimetric LAMP: Visual Detection for Simple Diagnostics (2017)
- Genome filtering identifies species-specific DNA biomarkers for Mansonella perstans and Mansonella ozzardi, which enable differentiation of these closely related species and other co-endemic filarial parasites (2019)
Feature Articles
Brochures
Web Tools
Posters
Products and content are covered by one or more patents, trademarks and/or copyrights owned or controlled by New England Biolabs, Inc (NEB). The use of trademark symbols does not necessarily indicate that the name is trademarked in the country where it is being read; it indicates where the content was originally developed. The use of this product may require the buyer to obtain additional third-party intellectual property rights for certain applications. For more information, please email busdev@neb.com.
This product is intended for research purposes only. This product is not intended to be used for therapeutic or diagnostic purposes in humans or animals.