Purification of RNA from the Aqueous Phase Following TRIzol®/Chloroform Extraction using the Monarch® RNA Cleanup Kits
RNA isolation reagents containing guanidine thiocyanate and phenol (e.g., TRIzol, RNAzol®, QIAzol®, etc) combined with chloroform extraction, are often used for sample lysis and RNA purification. The aqueous phase from any guanidinium thiocyanate-phenol-chloroform extraction can be cleaned up using the Monarch RNA Cleanup Kits (NEB #T2030, T2040, T2050), thereby eliminating the need for tedious RNA precipitation steps.
Before You Begin:
- Add 4 volumes of ethanol (≥ 95%) to the Monarch RNA Wash Buffer before use, as directed on the bottle.
- All centrifugation steps should be carried out at 16,000 x g. (~13K RPM in a typical microcentrifuge). This ensures all traces of buffer are eluted at each step.
Protocol
- Following guanidinium-thiocyanate-phenol-chloroform extraction,
carefully transfer the upper aqueous phase into an RNase-free tube (not
provided).
- Add 1 volume of ethanol (≥ 95%). Mix well by pipetting up and down or
flicking the tube. Do not vortex.
- Insert an RNA cleanup column into a collection tube, load sample onto the
column and close the cap. Spin for 1 minute, then discard flow-through.
For diluted samples ≥ 900 μl, load a portion of the sample, spin, and then
repeat as necessary.
 To save time, spin for 30 seconds, instead of 1 minute.
To save time, spin for 30 seconds, instead of 1 minute.
- Re-insert the column into the collection tube. Add 500 μl RNA Cleanup
Wash Buffer and spin for 1 minute. Discard the flow-through.
 To save time, spin for 30 seconds, instead of 1 minute.
To save time, spin for 30 seconds, instead of 1 minute.
- Repeat wash (Step 4).
- Transfer the column to an RNase-free 1.5 ml microfuge tube (not
provided). Use care to ensure that the tip of the column does not come
into contact with the flow-through. If in doubt, re-spin for 1 minute to
ensure traces of salt and ethanol are not carried over to next step.
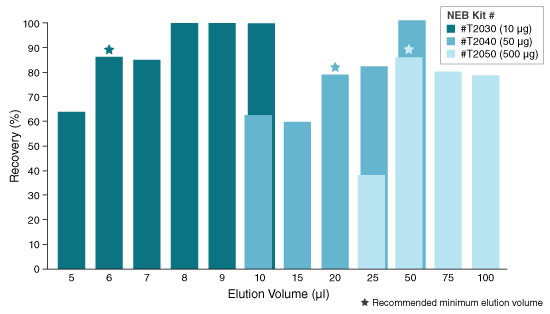
- Elute in nuclease-free water according to the table below. The eluted RNA can
be used immediately or stored at -70ºC. Care should be used to ensure the
elution buffer is delivered onto the center of the matrix and not the wall of the
column to maximize elution efficiency.
KIT ELUTION VOLUME INCUBATION TIME SPIN TIME T2030 6–20 µl N/A 1 minute T2040 20–100 µl N/A 1 minute T2050** 50–100 µl 5 minutes (Room temp.) 1 minute
* When cleaning up large amounts of RNA (> 100 μg, NEB #T2050), some precipitation may occur following the addition of the Monarch RNA Cleanup Binding Buffer and ethanol to the sample (Steps 1 and 2). A pellet containing the RNA of interest may form on the side of the column following the first binding spin (Step 3). To maximize recovery of this RNA, a second elution is recommended.
** Yield may slightly increase if a larger volume is used, but the RNA will be less concentrated.
 To save time, spin for 30 seconds, instead of 1 minute.
To save time, spin for 30 seconds, instead of 1 minute.
Additional Resources:
- Monarch RNA Cleanup Kit Protocol
- Product Manual
- Troubleshooting Guide for RNA Cleanup
- Visit NEBMonarch.com for information on these and other Monarch Kits

